|
FUEL CELLS
Please use our A-Z INDEX to navigate this site where page links may lead to other sites, or see HOME
|
|
|
The Fuel Cells and Hydrogen Undertaking (FCH JU) are working to facilitate the market introduction of FCH technologies in Europe. They do this by implementing research and innovation (R&I) programmes in order to develop a portfolio of clean, efficient solutions that exploit the properties of hydrogen as an energy carrier and fuel cells as energy converters. They are not concerned with integration into a national infrastructure, just the enabling (seed) dots so that policy makers may join them.
The invention of fuel cells was as important as the solar cell in terms of a giant leap for mankind aiming for renewable energy and sustainability of the planet in the creation of a circular economy.
We
need to head towards a circular economy at warp speed, to head off rising
temperatures, casing global
warming and mass
extinctions on planet earth.
In 1932, English engineer Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell. The alkaline fuel cell (AFC), also known as the Bacon fuel cell after its inventor, is one of the most developed fuel cell technologies, which NASA has used since the mid-1960s.
Three years later another GE chemist, Leonard Niedrach, devised a way of depositing platinum onto the membrane, which served as catalyst for the necessary hydrogen oxidation and oxygen reduction reactions. This became known as the "Grubb-Niedrach fuel cell".
GE went on to develop this technology with NASA and McDonnell Aircraft, leading to its use during Project Gemini. This was the first commercial use of a fuel cell. In 1959, a team led by Harry Ihrig built a 15 kW fuel cell tractor for Allis-Chalmers, which was demonstrated across the U.S. at state fairs. This system used potassium hydroxide as the electrolyte and compressed hydrogen and oxygen as the reactants.
Later in 1959, Bacon and his colleagues demonstrated a practical five-kilowatt unit capable of powering a welding machine. In the 1960s, Pratt & Whitney licensed Bacon's U.S. patents for use in the U.S. space program to supply electricity and drinking water (hydrogen and oxygen being readily available from the spacecraft tanks).
In 1991, the first hydrogen fuel cell automobile was developed by Roger Billings.
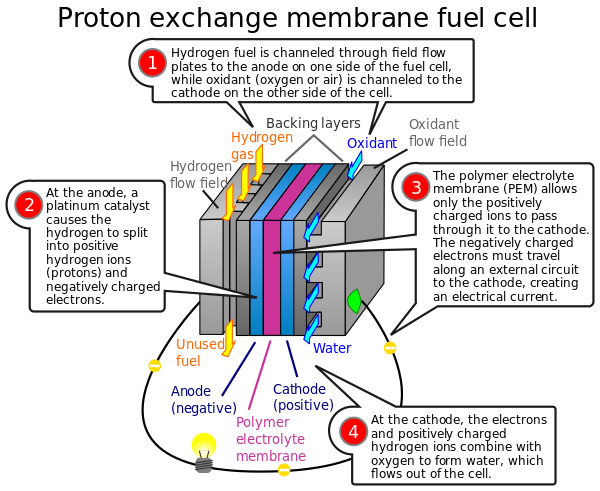
FUEL CELL CHEMISTRY
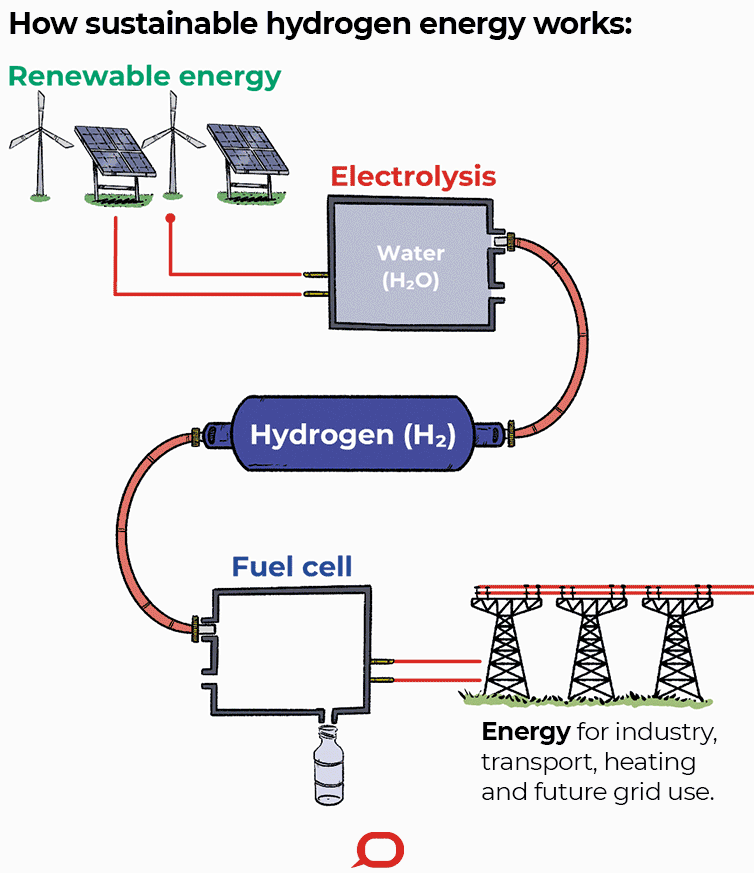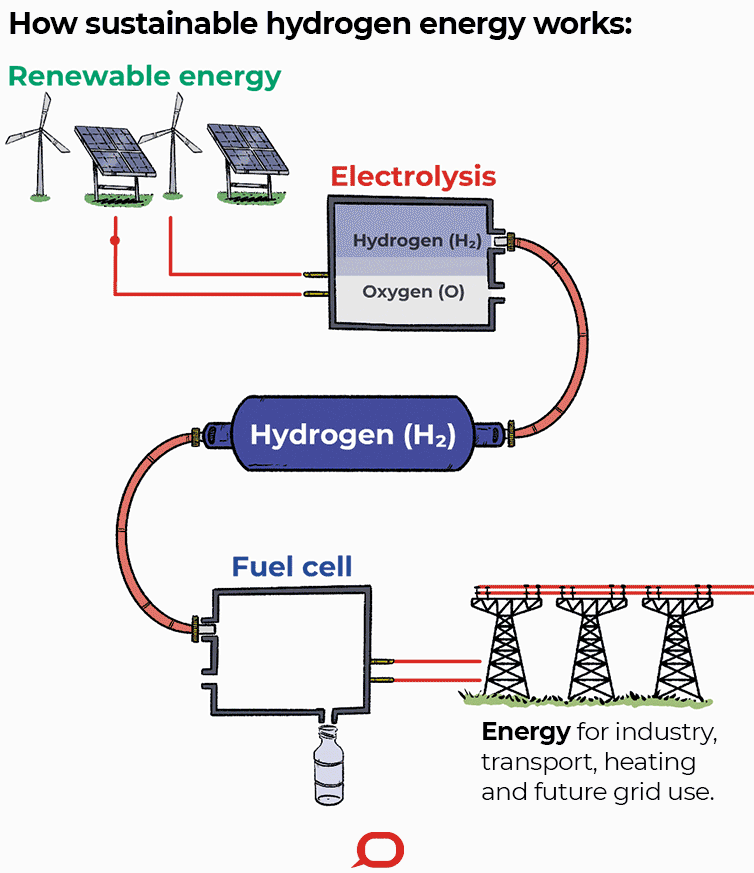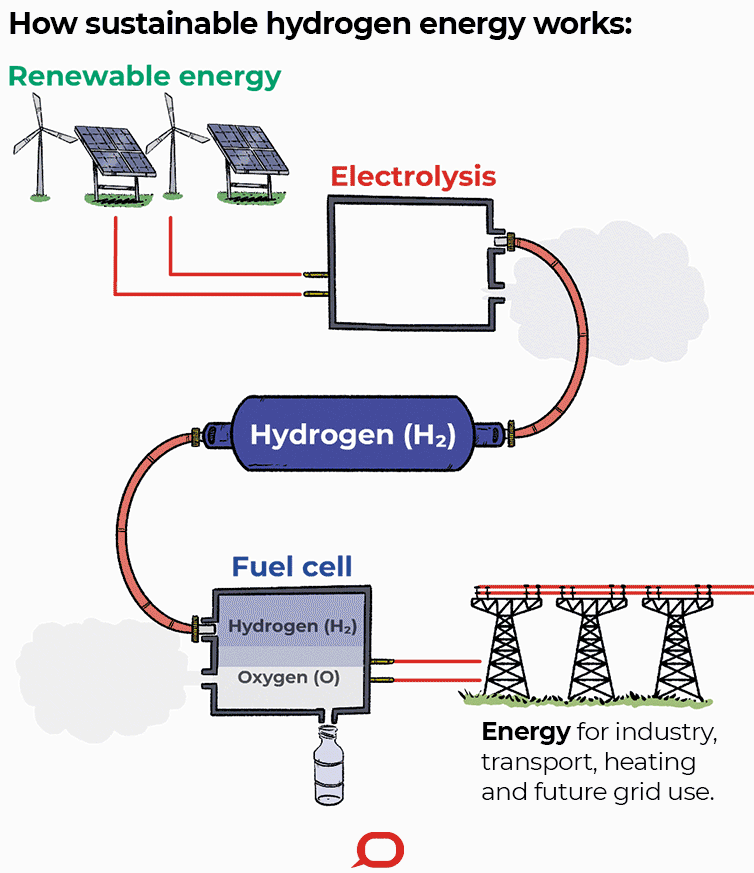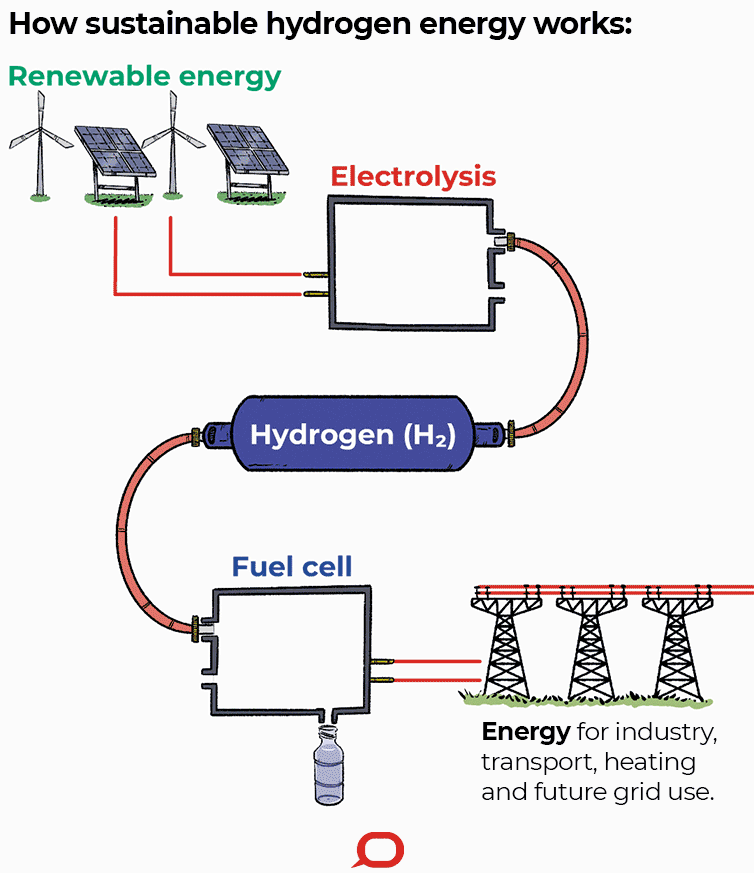
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from metals and their ions or oxides that are commonly already present in the battery, except in flow batteries. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
Fuel cells are classified by the type of electrolyte they use and by the difference in startup time ranging from 1 second for proton exchange membrane fuel cells (PEM fuel cells, or PEMFC) to 10 minutes for solid oxide fuel cells (SOFC). A related technology is flow batteries, in which the fuel can be regenerated by recharging. Individual fuel cells produce relatively small electrical potentials, about 0.7 volts, so cells are "stacked", or placed in series, to create sufficient voltage to meet an application's requirements. In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40–60%; however, if waste heat is captured in a cogeneration scheme, efficiencies of up to 85% can be obtained.
Automotive engines must also be able to start reliably at −30 °C (−22 °F) and have a high power-to-volume ratio (typically 2.5 kW/L).
US
NATIONAL HYDROGEN & FUEL CELL DAY
The most common fuel cell is comprised of a stack of PEM modules.
FUEL CELL & HYDROGEN NEGATIVES
We need to be realistic and recognise that fuel cells used with hydrogen may not be as efficient as using renewable energy directly, wherever this is possible. Hence, we must use solar and wind power directly when we can.
If you take a solar panel and use the energy from that to charge a conventional lithium battery pack directly, compared to trying to split water, take the hydrogen, dump the oxygen, compress the hydrogen to an extremely high pressure (or liquefy it) and then put it in a truck or ship and run a fuel-cell, the conversion chain is about half the efficiency of the lithium battery scenario.
What
a waste. In climate change terms why would you do that? It seems to make no
sense, except that there is a theoretical surplus of generating energy from
wind turbines, and hydrogen
provides better ranges for electrically powered vehicles.
Even so, the hydrogen battery is useful in the search for a circular economy, where lithium batteries may not provide for sustainable transport as the minerals needed are exhausted.
Hydrogen powered electric buses are becoming very popular. With exchange refuelling using high pressure gas cartridges, or liquid hydrogen cartridges, coaches and trucks might have unlimited ranges. We hope this is a topic of discussion at the forthcoming UN COP 26 in Glasgow, Scotland in November 2021.
JULY 2015 - Two electric aircraft crossed the English Channel, just a day apart. The performance of the Airbus E-Fan could be doubled using hydrogen batteries.
CHANNEL HOPPER - Electric ferries and river boats like the proposal above, could supplement solar and wind power, with hydrogen batteries to boost performance and reduce transit times, with refuelling at each end of a journey - but also with renewable performance of around 10 knots in reserve. Many fleet operators are now looking to hydrogen as a long term solution. Hydrogen batteries could be stacked for such endeavour.
LINKS & REFERENCE
https://www
Please use our A-Z INDEX to navigate this site
This website is provided on a free basis as a public information service. copyright © Climate Change Trust 2021. Solar Studios, BN271RF, United Kingdom.
|